Recently, I was working on a custom API program for a customer, when I came across something that could be a sticky little problem for someone trying to add custom API programs to their PTC Integrity Lifecycle Manager Environment. The problem was first manifested...
PLM
5 Reasons You Want Your PLM in the Cloud
Put it in the cloud. You’ve heard this catch phrase over and over again, more so in recent years with the proliferation of online technologies and services. As more and more PLM software vendors choose to move their application to the cloud, should you consider moving...
ROHS 2 for Medical Devices: Are You Ready?
As of July 22, 2014, the RoHS (Restriction of Hazardous Substances) Directive must be observed for first time distribution of all medical devices to the full extent. Furthermore, all products with a CE marking must also be RoHS-compliant. ROHS 2 Compliance Changes at...
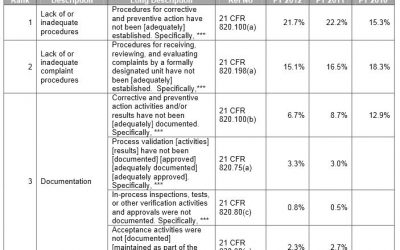
FDA Form 483: Top Ten Observations for Medical Devices
Medical Device manufacturers regulated by the FDA are subject to cGMP (Current Good Manufacturing Practice) regulations and may be inspected by the FDA to ensure compliance. If the FDA inspector(s) observes conditions that in their judgment may constitute violations,...
Integrating Medical Device Product Development with the Quality Management System
A critical business challenge for medical device manufacturers as they scale is getting products to market quickly while supporting existing products and meeting FDA Quality System Regulation (21-CFR-820) requirements. To achieve this effectively, Product Development...
The Convergence of ALM and PLM in the Technology Industry
As more and more technology is embedded into products, and software continues its role as the primary driver for product innovation, the domains of application lifecycle management (ALM) and product lifecycle management (PLM) are being placed on an inevitable...
Where Does ALM fit into the World of PLM?
Historically, Product Lifecycle Management (PLM) has its roots in mechanical and electrical engineering, specifically the automotive industry. Its use was pioneered to manage a product (for instance, a car) from inception and on through computer-aided design (CAD),...
PLM: Automate your Product Development Compliance Process
Developing new and innovative products is essential for companies to survive and thrive -- however safety can never take a backseat to innovation. That is why many companies, like those in the Medical Devices, Aeronautics and Automotive industries, have strict...
How PLM Enables Innovation Without Risking Compliance
As technology has advanced, it's become more complex -- and with complexity comes the issue of tracking and monitoring the different processes and their corresponding data. In non-critical applications, the extent of this reporting is often minimal. However, in...
Is the Cloud the Best for Application Management Services?
As the push towards the cloud continues, the inertia of its popularity can leave CTOs with the impression that the world is black and white with only two options: in the cloud or not in the cloud. The nuances and diversity of solutions can therefore be lost in the...
Defining PDM and PLM Software: Why You Want Both
Choosing and evaluating products for managing data is a daunting task. With the ease of access to broad, general information – which is often conflicting – getting your decision-makers and Very Important People using the same vocabulary is the first step to developing...
How to Solve Costly Monotony and Redundancy with Automation
Redundant and repetitive tasks present a number of challenges for your business. Your engineering team feels bored, like they’re wasting their time. Pretty soon, they’re done being bored and looking for more challenging and rewarding work. With skilled engineers in...