Medical Device manufacturers regulated by the FDA are subject to cGMP (Current Good Manufacturing Practice) regulations and may be inspected by the FDA to ensure compliance. If the FDA inspector(s) observes conditions that in their judgment may constitute violations, they will issue a Form 483 to the firm management at the conclusion of the inspection.
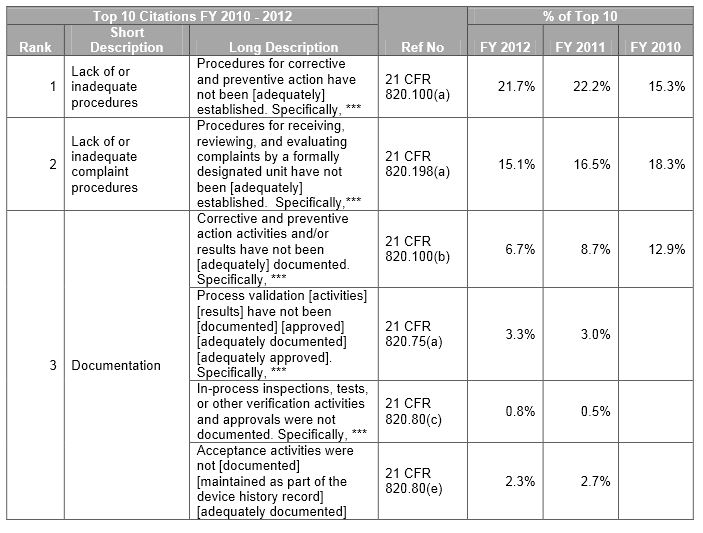
The FDA has issued over a thousand 483 forms yearly for medical devices over the past three years. We review the top 483 citations from FY 2010 – 2012 and show how an efficient product lifecycle management (PLM) environment can help avoid these issues.
Below is a sample. Download our whitepaper for the full view of the top ten observations.
Next Steps:
- Contact SPK and Associates to see how we can help your organization with our ALM, PLM, and Engineering Tools Support services.
- Read our White Papers & Case Studies for examples of how SPK leverages technology to advance engineering and business for our clients.