The release of the FDA final rule on Unique Device Identification (UDI) is expected this summer. Here are five things you need to know:
1. What is the UDI Rule?
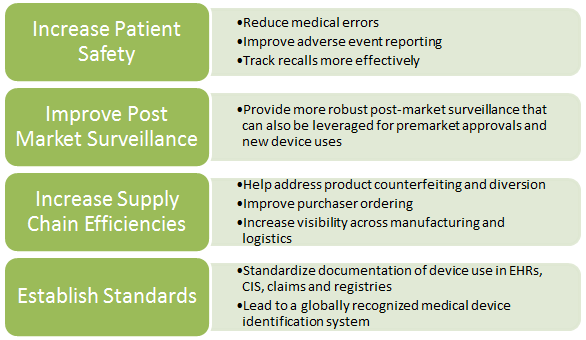
In July 2012, the FDA proposed a rule requiring medical device manufacturers to label their products with unique device identifiers (UDIs). The final rule is under Office of Management and Budget review and is expected to issue this summer. It would establish a unique device identification system for tracking medical devices to:
2. What is the Unique Device Identifier?
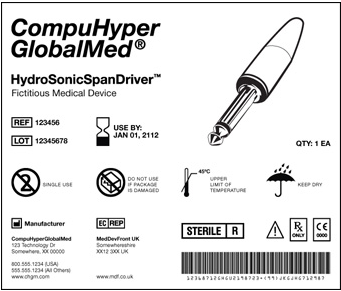 The UDI is a unique numeric or alphanumeric code that includes:
The UDI is a unique numeric or alphanumeric code that includes:
- A Device Identifier (DI): [static] Manufacturer, make, model [i.e., each catalog number]
- A Production identifier (PI): [dynamic] how product is currently controlled — serial, lot number; expiration, manufacturing date
The UDI must appear on the label in a human readable format, that can be read by automatic identification and data capture (AIDC) technology, such as a linear or 2D DataMatrix barcode. A unique UDI must be applied to the “base package” and higher levels of packaging.
The UDI will be submitted to the FDA Global UDI Database (GUDID) and include a standard set of basic identifying data attributes for each UDI.
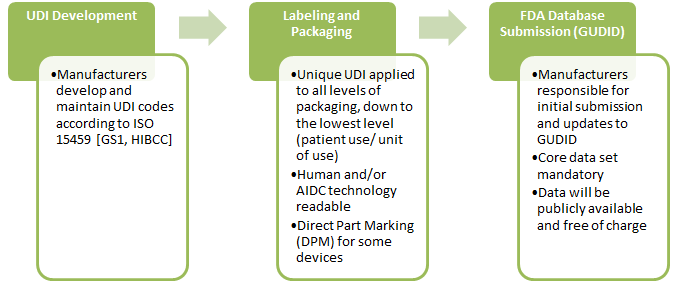
3. How will the UDI system be implemented?
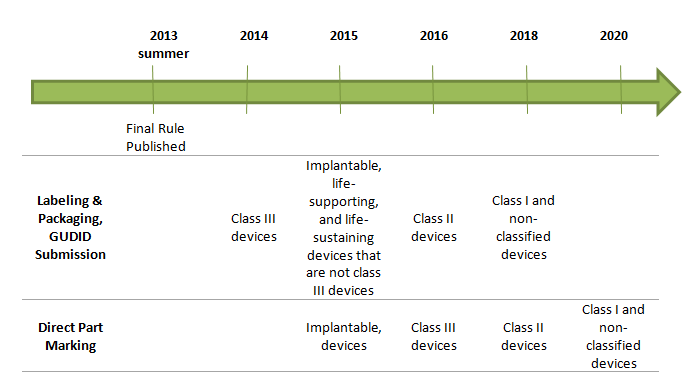
4. What is the timeline for implementation?
The anticipated effective dates for UDI requirements are based on risk class after publication of final rule:
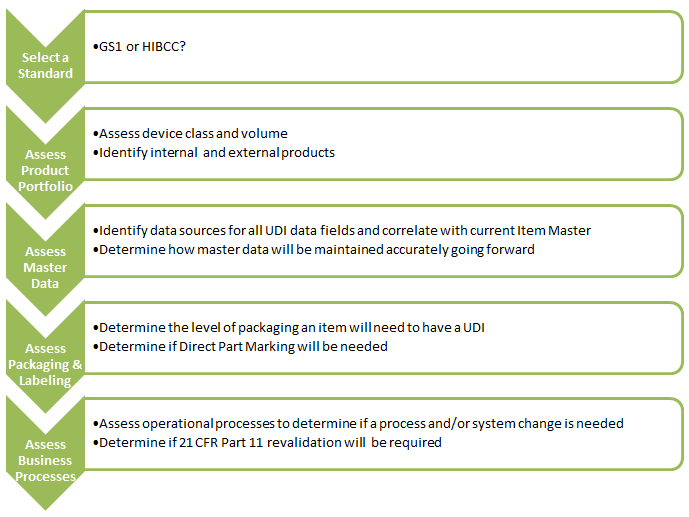
5. What are the immediate next steps for medical device manufacturers?
If your UDI efforts are not underway already get started now! The immediate next steps are:
Where can I find more information?
- FDA UDI Guidance: http://www.fda.gov/medicaldevices/deviceregulationandguidance/uniquedeviceidentification/default.htm
- GS1: http://www.gs1us.org/industries/healthcare/gs1-healthcare-us/fda-udi
- HIBCC: http://www.hibcc.org/udi-labeling-standards/
- PRISYM ID webinar: http://www.prisymid.com/about-us/events/14-webinars/260-tips-for-implementing-fda-udi-without-derailing-production
Next Steps:
- Contact SPK and Associates to see how we can help your organization with our ALM, PLM, and Engineering Tools Support services.
- Read our White Papers & Case Studies for examples of how SPK leverages technology to advance engineering and business for our clients.