The journey from concept to market-ready medical device is no easy feat. The regulatory economy, development and legislation is intricate. Understandably, compliance with standards is non-negotiable to ensure the safety and efficacy of medical devices. One such standard is ISO 14971:2019, the international standard for risk management in medical devices. In this comprehensive guide, we’ll navigate the regulatory landscape, explore the intricacies of ISO 14971 compliance, and shed light on the role of innovative tools to streamline and simplify compliance to legislation.

The Risk Management Lifecycle
In the risk management lifecycle, the journey starts with hazard analysis, evaluating potential failure or accident scenarios. Risk identification follows, providing a detailed assessment of adverse events and their impact. Classification and assessment categorize risks per industry guidelines to ensure market fitness. Moving to risk mitigation, controls are identified to minimize risks, ranging from product features to inspections. The subsequent step involves structuring controls into an actionable plan, assigning responsibilities. Finally, documentation, reports, and dashboards play a key role in monitoring and providing evidence of effective risk management practices.
Risk mitigation and reduction planning correspond to ISO 14971’s focus on implementing controls to manage risks. Additionally, the emphasis on documentation and reporting in the lifecycle aligns with ISO 14971’s requirement for comprehensive documentation, ensuring adherence to regulatory standards throughout the risk management process in the medical device industry.
Understanding ISO 14971:2019
ISO 14971:2019 is not just a set of guidelines; it is the consensus standard recognized by the FDA and harmonized with the EU MDR. And, at its core, this international standard outlines a comprehensive framework for managing risks associated with medical devices. The requirements specified in ISO 14971 require manufacturers to:
-
- Identify hazards linked to their medical devices.
- Analyze these risks meticulously.
- Plan and execute measures to mitigate or reduce these risks.
- Monitor and assess the effectiveness of these risk mitigation efforts.
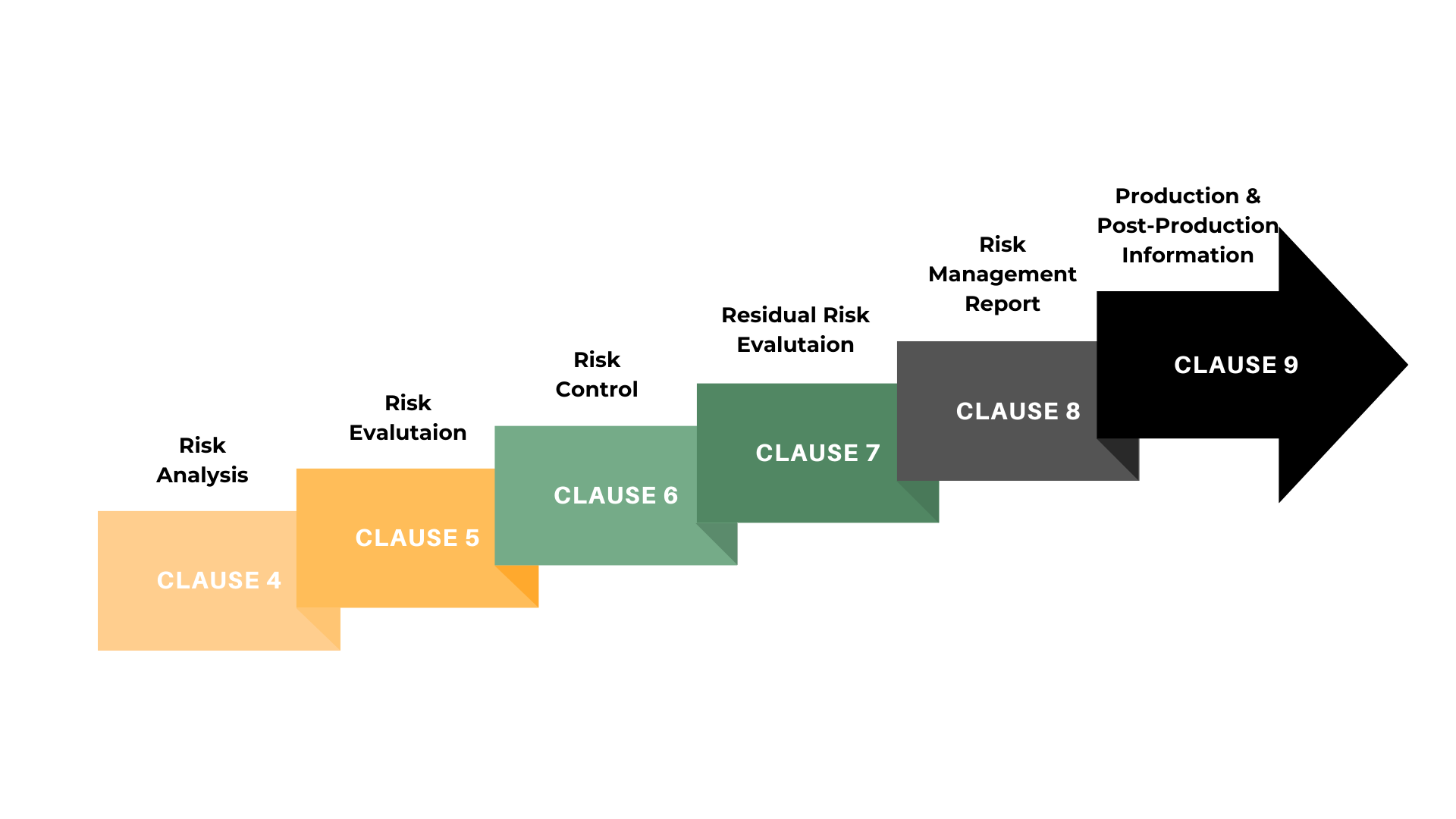
Challenges in Medical Device Risk Management
However, the journey to regulatory clearance encounters challenges necessitating undeniable risk management processes. For example, two major hurdles include:
- Tooling for Collaboration: Traditional methods involving extensive Excel sheets with numerous risk entries lack versioning and traceability, hindering collaborative work. Therefore, this poses difficulties in creating a risk matrix and linking risk mitigation to design controls.
- Organizational Misalignment: When each business unit adopts its standard operating procedure (SOP), organizational misalignment occurs. Thus, different interpretations, terminologies, and tools lead to siloed information access. Ultimately, this complicates risk management.
Solutions To Support ISO 14971 Compliance
The Role of Codebeamer in Risk Management
Codebeamer has a wealth of benefits. In fact, we published a blog in early 2023 where you can learn the benefits of Codebeamer in five minutes. But, for ease, here are the best ways Codebeamer can support you to remain compliant with ISO14971.

1. Comprehensive Lifecycle Adherence
- Integrated Solution: Codebeamer provides a unified platform for requirements, risk, and test management, ensuring seamless integration with day-to-day activities throughout the product lifecycle.
- Holistic Approach: The solution promotes a comprehensive approach to risk management, addressing all stages of product development to enhance safety and compliance.
2. Robust Risk Registry
- Identification, Analysis, and Mitigation: Codebeamer empowers teams to create a robust risk registry, facilitating the identification, analysis, and mitigation of hazards and risks.
- ISO Compliance: The solution aligns with ISO 14971 and other safety-critical regulations, providing a standardized framework for risk management.
3. Documentation and Management
- CAPA, FMEA, and More: Codebeamer enables the documentation and management of CAPA, FMEA, and other risk-related activities, streamlining processes and enhancing traceability.
- Regulatory Confidence: The solution empowers you to respond to regulatory audits with confidence, providing a transparent and auditable trail of risk management activities.
4. Closed-Loop Integration
- Engineering Digital Thread: Codebeamer facilitates closed-loop integration with the PTC engineering digital thread, ensuring a seamless flow of information across engineering processes.
- Efficiency and Collaboration: By connecting different facets of the product development lifecycle, Codebeamer enhances efficiency and collaboration. Ultimately, this contributes to a more streamlined and effective risk management process.
5. Cultivating a Culture of Safety and Quality
- Enduring Principles: Codebeamer goes beyond being a tool; it helps medtech companies to build a culture of safety and quality. And, by providing the necessary framework and tools, it supports the adoption of enduring risk management principles throughout the organization.
- Long-Term Impact: The solution’s focus on safety and quality contributes to the long-term success and reputation of the organization. Basically this helps risk management remain a central tenet of operations.
The Role of Greenlight Guru In ISO 14971
Greenlight Guru is designed explicitly for medical device manufacturers. Additionally, Greenlight Guru offers a suite of tools and resources to streamline compliance and product launches. Plus, with handy integrations to platforms like Jira, you can further enhance your team’s efficiency. Here are the best ways Greenlight Guru helps you remain compliant with ISO14971:

Cutting-Edge Risk Solutions
Greenlight Guru’s Risk Solutions are a new era in risk management for the MedTech industry. Powered by AI-generated insights and purpose-built risk management workflows, these solutions provide a streamlined path to compliance. Plus, they reduce risk throughout the entire device lifecycle.
Risk Management Module
The Risk Management module offers visually intuitive and collaborative workflows for creating risk matrices and documenting risks, aligning seamlessly with ISO 14971:2019. Additionally, it ensures complete traceability throughout the device lifecycle, addressing the intricacies of risk identification, analysis, and mitigation.
Risk Intelligence Module
Leveraging advanced statistical models, the Risk Intelligence module identifies the most relevant device hazards and patient harms based on real-world adverse event data. And, with AI-generated insights, it transforms the way MedTech teams work. This drives efficiency and confidence in risk management.
Linking Design Controls with Risk Management
In medical device development, the synergy between Design Controls and Risk Management is undeniable. That’s why Greenlight Guru’s Risk Solutions facilitate this connection, allowing teams to demonstrate a risk-based approach to design. The link-ability and full traceability to related design controls and components ensure a cohesive and integrated product development process.
Living Risk Management Throughout the Product Lifecycle
While many companies face challenges in keeping their Risk Management File (RMF) a living document after product development, Greenlight Guru’s software provides a solution. Offering in-line editing, auto-calculated risk probabilities, and up-to-date codes from IMDRF, Essentially, Greenlight Guru ensures that your RMF remains readily available for updates with production and post-production information.
Connecting Production and Post-Production with Risk Management
Greenlight Guru goes beyond the development phase, emphasizing the importance of Risk Management throughout the total product lifecycle process. It encourages companies to consider and document production-related risk management activities and events. That means it ensures post-production processes seamlessly integrate with the Risk Management process.
A Holistic Approach to Risk Management Review
As you approach the final stages of bringing a medical device to market, Greenlight Guru supports a holistic Risk Management Review. The Risk Management Report, summarizing all risk management activities and benefit-risk analyses, receives executive management approval. Ultimately, this provides a comprehensive overview pre-market entry.
Check out the top 10 reasons to use Greenlight Guru for your medical device development.
In Summary
Navigating the complexities of the journey from medical device concept to market readiness requires strict adherence to standards like ISO 14971:2019. In this guide, we’ve outlined how using tools like Codebeamer and Greenlight Guru can help you streamline your compliance. Codebeamer ensures lifecycle-wide adherence, offering a robust risk registry and closed-loop integration. Greenlight Guru’s Risk Solutions align with ISO 14971, emphasizing cohesive product development, living risk management, and holistic risk reviews. These tools, addressing collaboration challenges, contribute to a streamlined and effective risk management process.
If you need support accelerating your medical device time to market, or you want to get started with Codebeamer and Greenlight Guru, we can help. SPK are proud partners of both PTC Codebeamer and Greenlight Guru, so we’re perfectly positioned to make them work for you. Plus, we’ve already helped medtech businesses globally, to achieve compliance and market-perfect product delivery.







