The FDA plays a pivotal role, setting the gold standard for regulatory oversight. Manufacturers must meticulously adhere to FDA regulations, such as the Quality System Regulation (QSR) under 21 CFR Part 820, governing key aspects like design controls, production processes, and post-market surveillance. Furthermore, beyond the FDA, global harmonization efforts aim to standardize regulatory expectations, facilitating smoother market access for manufacturers on an international scale. The Device History Record (DHR), Device Master Record (DMR), and Design History File (DHF) are the trifecta of medical device documentation compliance. These three components form a backbone ensuring the safety, efficacy, and regulatory compliance of medical devices. This blog post is your introduction to the basics of the trio DHF, DMR and DHR.
Impact of Non-Compliance And How DHF, DMR, DHR Can Help
The consequences of non-compliance with documentation compliance are far-reaching. For example, regulatory sanctions, including warning letters and fines, can disrupt market entry and tarnish a manufacturer’s reputation. Additionally, product delays, withdrawals, and legal actions may follow, imposing financial burdens and eroding consumer trust. Therefore, as medtech innovations continue to advance, staying abreast of regulations is non-negotiable. Legislative changes, like the European Union’s Medical Device Regulation (MDR), reshape compliance frameworks and manufacturers must adapt swiftly to meet these shifts.
Strategies for Success: DHF, DMR, DHR Medical Documentation
Effective compliance strategies involve establishing robust Quality Management Systems (QMS), incorporating design controls, and meticulously documenting the trio of DHF, DMR, and DHR medical documentation.

Check out these tips for a successful eQMS implementation.
Regular audits, continuous training, and leveraging purpose-built quality management software contribute to a proactive culture of compliance. Now, let’s deep dive into the difference between DHF, DMR and DHR in medical documentation.
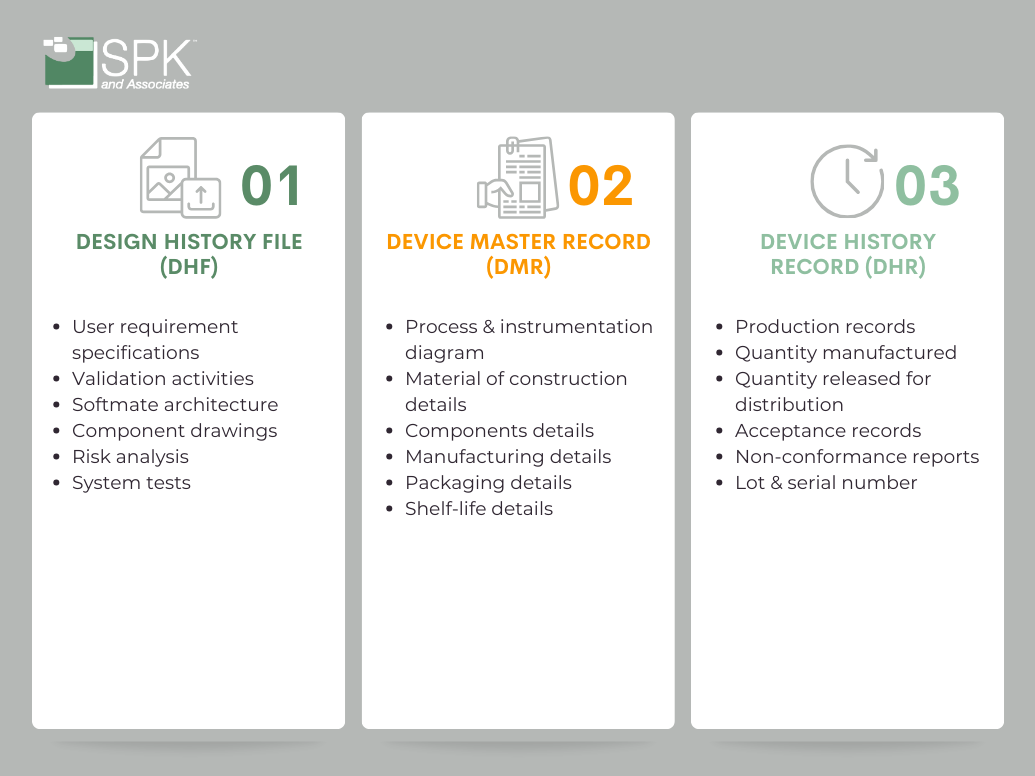
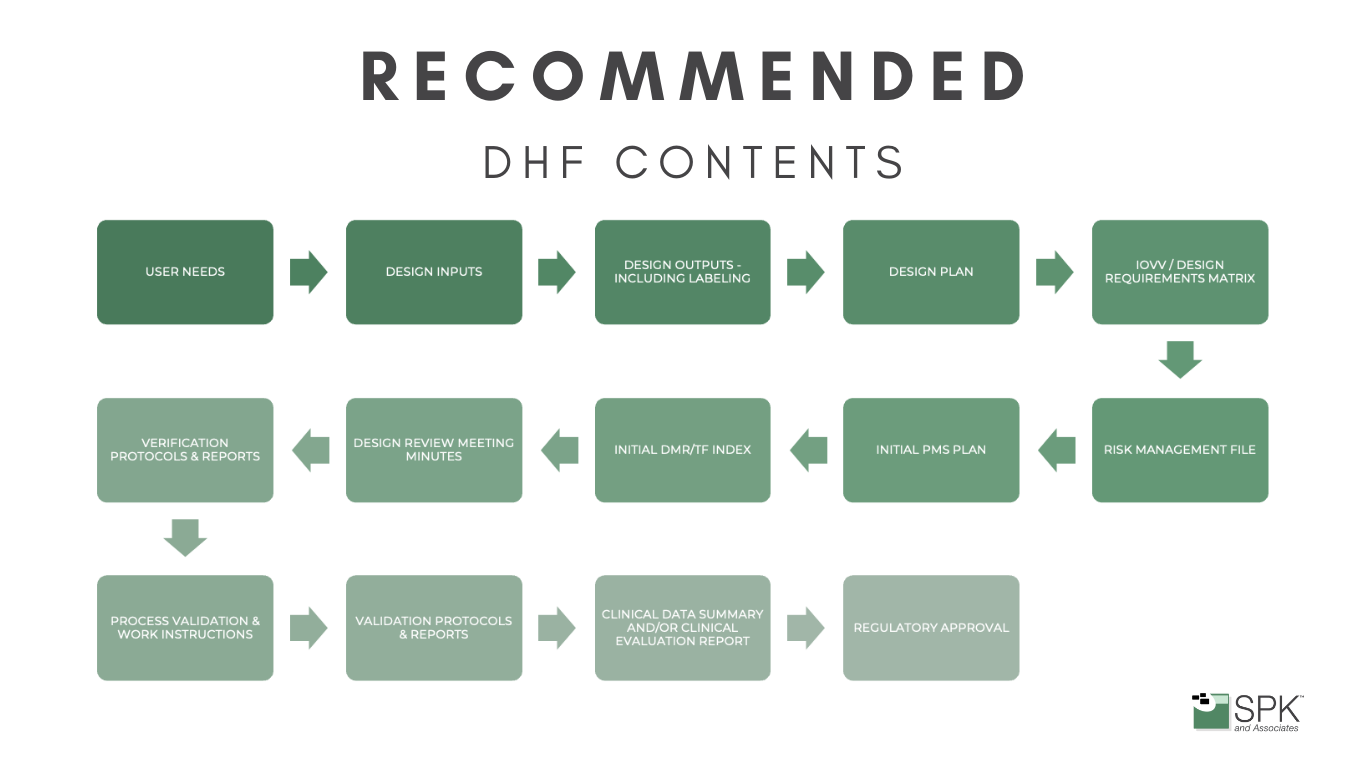
Design History File (DHF)
What is DHF and Why Does it Matter?
When you’re developing a medical device you’ll start by defining user needs and design inputs, which find their place in the DHF. As you progress, generating design outputs, creating verification and validation protocols, and undergoing design reviews, each step needs documenting. The DHF becomes the roadmap that ensures your design aligns with regulatory expectations.
Regulatory bodies, including the FDA, mandate the DHF as a repository of evidence, substantiating that your medical device adheres to sanctioned plans and complies with rigorous regulatory standards. Essentially, the DHF becomes not just a record but a strategic asset, ensuring your innovations align seamlessly with regulatory expectations and industry best practices.
Maintaining a well-organized DHF is not just a regulatory obligation; it’s a practical necessity. Auditors and inspectors will scrutinize this file to verify compliance, making organization crucial. Therefore, creating a living document that evolves alongside the design and development process simplifies the task, ensuring no detail is overlooked.
What’s Inside the DHF?
The DHF is a comprehensive snapshot of the entire design journey. It includes:
- Initial user needs and design inputs.
- Design outputs that detail device specifications.
- Verification and validation protocols, and reports.
- Records of design reviews.
The Device Master Record (DMR)
What is DMR and Why Does it Matter?
Once the design phase is complete, it’s time to transition from the design of the device to the development. So, this is where the Device Master Record (DMR) steps in. It’s the master guide for production, offering detailed specifications and instructions for manufacturing a specific type of medical device.
The DMR ensures consistency in production. Basically, it guides manufacturing processes to meet the specifications outlined during design. Furthermore, compliance with DMR specifications is a regulatory requirement and contributes to the overall quality and reliability of the medical device.
What’s The Difference Between DMR and DHF?
While the DMR may sound like a duplicate of the DHF, it serves a distinct purpose.
- Think of the DHF as the history of the design.
- The DMR is the detailed instructions for building and testing the device.
The beauty lies in the connection between them; the DMR references the necessary information from the DHF without duplication.
What’s Inside the DMR?
Essentially, the DMR is the comprehensive set of instructions for building, testing, packaging, and servicing the device. The DMR contains:
- Device specifications.
- Production process specifications.
- Quality assurance procedures.
- Packaging and labeling specifications.
- Procedures for installation, maintenance, and servicing.
The Device History Record (DHR)
What is DHR and Why Does it Matter?
As your device comes to life on the production line, the Device History Record (DHR) takes center stage. It’s the detailed record of each device’s journey from production to distribution, serving as the conclusive chapter in the trilogy of documentation.
Traceability is key in manufacturing, and the DHR provides a traceable history for each unit. It ensures accountability by documenting that each device was manufactured according to the specifications outlined in the DMR. This meticulous record is essential during regulatory inspections.
What’s Inside the DHR?
The DHR captures critical information such as dates of manufacture, quantity produced, quantity released for distribution, acceptance records confirming compliance with the DMR, and identification labels for each unit. It’s the history book of each device’s creation.

How Does A Good eQMS Support DHF, DMR and DHR Medical Documentation Compliance?
An Electronic Quality Management System (eQMS) like Greenlight Guru greatly enhances the management of documentation in medical device manufacturing. For example, by providing a centralized repository, it ensures stakeholders access the most current and accurate documents, avoiding version control issues. Furthermore, robust document control features maintain compliance, allowing only approved and current versions to be accessible. Automated workflows also streamline document processes, from creation to approval, reducing delays and improving efficiency. Integration with design controls also ensures interconnectedness of documents related to design inputs, outputs, verification, and validation. Lastly, electronic signatures, collaboration features, and detailed version histories further contribute to maintaining good documentation practices.
Greenlight Guru is the only eQMS specifically designed for medical device manufacturers. And, we have a special offer on Greenlight Guru for you. Check out the details here.
Conclusion
Understanding the DHF, DMR, and DHR is not just a compliance exercise; it’s a roadmap for ensuring the quality, safety, and regulatory adherence of medical devices. These documents form a connected trilogy, each playing a crucial role in the device’s lifecycle. Furthermore, regardless of your business size, you must embrace these documentation practices to ensure regulatory compliance.
If you need support to improve your documentation compliance procedures, or to migrate to a new eQMS, we can help. SPK partners with medtech manufacturers globally. Plus, we’re partners with MasterControl and Greenlight Guru and can support you to achieve regulatory compliance. We know the last thing you want is that dreaded FDA letter. Contact our team today to get started.







