In a year of unexpected twists, the medical technology (MedTech) industry finds itself amidst shifting sands. So, Greenlight Guru’s 2023 MedTech Industry Benchmark Report is a treasure trove of insights into MedTech trends and the landscape. Let’s deep dive into some of Greenlight Guru’s report findings.
Who is Greenlight Guru?
Before we dive in, we want to explain why Greenlight Guru’s report is fundamental to MedTech companies.

Greenlight Guru are leaders in the eQMS industry for MedTech companies. It’s one of the reasons we’re proud to partner with them.

Greenlight Guru’s Report Findings: Financial Conundrums
While the revenue forecasts show a gentle dip, with only 26% of firms predicting strong growth, it’s essential to keep perspective. Research from Frost & Sullivan indicates a slight decrease in growth rate from last year. But, remember MedTech has bounced back significantly since the 2020 pandemic.
Greenlight Guru’s Report Shows Pivoting Priorities
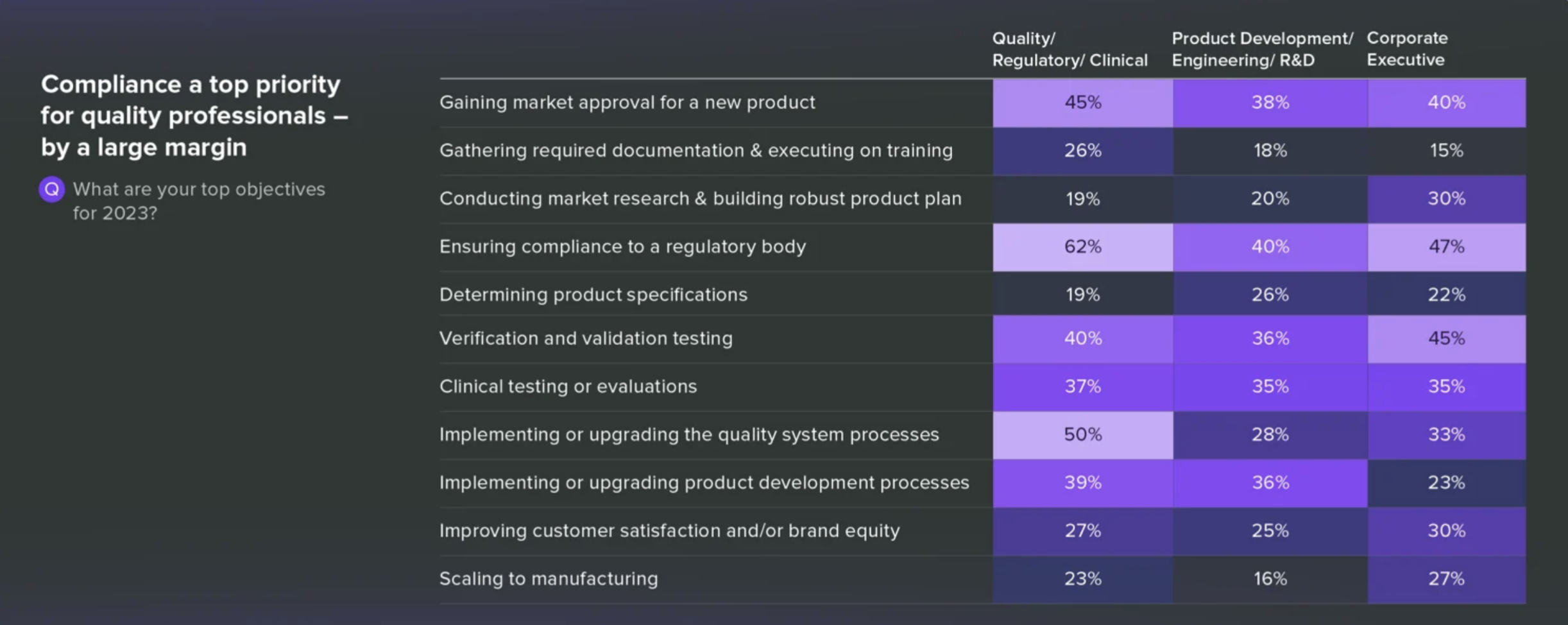
2022 was marked by the accelerated product development, fueled in part by the pandemic’s wane. 2023. However, it has the industry singing a different tune. Apparently, compliance is the new buzzword.
This shift in focus is further underscored by the revelation that a significant number of companies are working to revamp their quality system processes to stay compliant. It’s a prudent move, given the crucial role compliance plays in ensuring patient safety and maintaining industry standards.
MedTech Trends: Facing Realities and Harnessing Tech
A significant chasm exists between expectations and realities for pre-market MedTech companies. They’re underestimating commercialization timelines by a whopping 53%. This underestimation can be attributed to oversight in vital scaling activities, including employee training, hiring for manufacturing roles, and the need for robust engineering support.
On the technology front, Greenlight Guru’s report highlights there’s a massive drive for modernization.
Bridging Gaps and Investing Wisely
Challenges in documenting design controls, risk management processes, and ensuring complete traceability are still rife. There’s an undeniable need for cohesive tools that seamlessly blend these functionalities. Additionally, in-house training programs and courses are becoming indispensable, particularly for better understanding compliance requirements.So, You’ve Read Greenlight Guru’s Report Highlights…What Do You Need To Do Now?
Fundamentally, there’s a harder shift in MedTech trends towards compliance this year and a burgeoning emphasis on technological transformation. Greenlight Guru’s report is an invaluable resource, offering a comprehensive look at the current state of affairs and indicating the path forward.
Download Greenlight Guru’s report in full directly from the leaders themselves. You’ll get access to a deeper exploration and to tap into opportunities in 2023 and beyond.







