Developing new and innovative products is essential for companies to survive and thrive -- however safety can never take a backseat to innovation. That is why many companies, like those in the Medical Devices, Aeronautics and Automotive industries, have strict...
Medical Device Engineering
How PLM Enables Innovation Without Risking Compliance
As technology has advanced, it's become more complex -- and with complexity comes the issue of tracking and monitoring the different processes and their corresponding data. In non-critical applications, the extent of this reporting is often minimal. However, in...
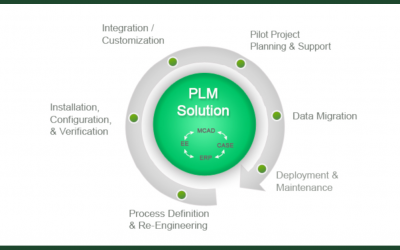
Accelerating Product Development with Engineering Operations
What is Engineering Operations (EngOps)? Why do you need it, and how do you effectively deploy it?There’s a strange interplay between IT and engineering teams in most firms. That’s because the tech stack that engineers need to design, build, test, and release products...
Difficulty of BOMs Across Engineering Disciplines (Part 1)
SPK and Associates co-founder Chris McHale speaks with PLM expert Oleg Shilovitsky, founder of BeyondPLM.com, to get his insight on the difficulty of BOMs across engineering disciplines.
The State of Digital Quality Maturity in Pharma and Medtech
Organizations in the medical, pharmaceutical, and life science industries are constantly adapting to their field’s rapidly changing technology and regulations. This continuous adjustment can become exhausting. Between this burnout and being unsure if your technology...
Introduction to ISO 14971:2019 for Medical Device Compliance
Navigating the complexities of ISO 14971:2019 can be challenging. But, with this eBook presented by SPK and Associates and PTC, you're taking the first step towards expertise in medical device compliance. What's In The ISO 14971:2019 eBook This comprehensive guide...
2022 Guide: Software as a Medical Device (SaMD)
What is software as a medical device? Software as a medical device, or SaMD is software that is intended for one or more medical purposes. This software performs those purposes without being part of a hardware medical device. SaMD devices also need to meet the...
Using Creo to Meet Stringent Regulatory Requirements in Medical Device Engineering
All medical device manufacturers understand that precision, safety, and regulatory compliance are vital in their industry. Without these precautions, individuals could be harmed. These strict requirements and extended life cycles often make the time between designing...
Achieving the Speed of Innovation and Maintaining Compliance in the Medical Device Industry
Innovation is essential for staying ahead of competition in all industries, but especially in healthcare. Staying up to date with new technologies and compliance needs can improve outcomes for patients. The best way to ensure compliance is by utilizing the proper...
Accelerating Medical Device Development with the Digital Thread
The medical device industry is one of the most regulated industries due to the importance of the products they manufacture. Efficiently producing high-quality products that comply with regulatory standards is the key to successful medical device manufacturing....
Building Resilience in Healthcare IT Infrastructure through Cybersecurity Strategies
Cybersecurity is important in every industry but is especially vital in healthcare. Safety, compliance, and data protection become more important than ever. With cyber attacks on the rise, protecting sensitive patient data has become a main priority. Let’s explore...
Creo Composites Design & Manufacturing Capabilities
Engineers typically have a few distinct materials to work with when designing and manufacturing parts. Composite design allows them to combine two or more varying materials to create a new one. This new material is often sustainable and used to design structures. This...