In the past, medical device design focused on a standalone unit or a group of a couple devices that worked together. While that was a great idea, the number of compatibility issues that could occur also blocked connectivity to other devices. With the stream of new...
Medical Device Engineering
OSS, Heartbleed, and the Impact on Medical Device Design
The use of open source software (OSS) within the medical device industry is a double edged sword. On one end of the spectrum, you have freely available code that is available for the world to scrutinize. In the process, one would hope that bugs are more easily...
Wearable Medical Devices: The Latest Medical Device Design Trend
As humans, we depend on feedback. Everyone likes feedback, whether a glance in the mirror, a friendly compliment or financial reward for a great job. Now thanks to the advancement of medical device design, high tech trends, and use of micro-computers, you can get...
Cyber Security in Medical Device Design
With the push by big technology players (Cisco, Google, Intel, etc.) towards connectivity in everyday devices, cyber security is becoming more and more crucial. This push is even seen in medical device design as the industry begins to move toward cloud-integrated and...
March 19th Medical Device Seminar: Ready for ROHS 2 in July 2014?
Device manufacturers have until this summer to ensure their devices comply with the EU's Restriction of Hazardous Substances Directive – commonly called the RoHS Directive – to be able to earn CE Marking. In short, no RoHS compliance, no CE Marking, no selling in the...
Agile Development in Regulated Environments – Part 1: Yes, it can work
The value system and practices that embody Agile Software Development have been around for well over a decade, and have been touted as having "crossed the chasm" by organizations such as the Agile Alliance, Gartner, and Forrester Research. Numerous studies indicate...
Medical Device Interoperability: A $30B opportunity?
Greater medical device interoperability and the adoption of commonly accepted standards could save the US in excess of $30B, suggests a West Health Institute report published in March. Lack of device interoperability creates significant waste and risk to patient...
Tackling Email Archiving Regulations
Whether or not your in a heavily regulated industry, having a solid email archiving solution is an absolute necessity. Email’s importance in the workplace for the past decade-and-a-half is similar to the need of having a dial tone in years past. In addition, email is...
ROHS 2 for Medical Devices: Are You Ready?
As of July 22, 2014, the RoHS (Restriction of Hazardous Substances) Directive must be observed for first time distribution of all medical devices to the full extent. Furthermore, all products with a CE marking must also be RoHS-compliant. ROHS 2 Compliance Changes at...
The FDA UDI Rule: 5 Things You Need to Know
The release of the FDA final rule on Unique Device Identification (UDI) is expected this summer. Here are five things you need to know: 1. What is the UDI Rule? In July 2012, the FDA proposed a rule requiring medical device manufacturers to label their products with...
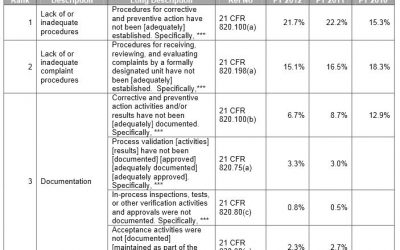
FDA Form 483: Top Ten Observations for Medical Devices
Medical Device manufacturers regulated by the FDA are subject to cGMP (Current Good Manufacturing Practice) regulations and may be inspected by the FDA to ensure compliance. If the FDA inspector(s) observes conditions that in their judgment may constitute violations,...
Integrating Medical Device Product Development with the Quality Management System
A critical business challenge for medical device manufacturers as they scale is getting products to market quickly while supporting existing products and meeting FDA Quality System Regulation (21-CFR-820) requirements. To achieve this effectively, Product Development...