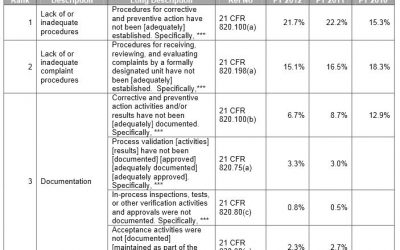
Medical Device manufacturers regulated by the FDA are subject to cGMP (Current Good Manufacturing Practice) regulations and may be inspected by the FDA to ensure compliance. If the FDA inspector(s) observes conditions that in their judgment may constitute violations,...
FDA 483 Observations
A Review of FDA 483 Observations – Top Med Device Issues Sited & Proper Response
This article reviews what an FDA 483 Observation looks like, some of the more common issues flagged in medical device companies, and how to respond.