About SPK and Associates
Headquartered in Scotts Valley, CA, SPK and Associates is a leading woman-owned Engineering & IT Services Company that serves product development teams. For over 20 years, we have been helping our customers to harness technology to optimize engineering and attain their business goals. We understand the systems, processes, data and applications critical to successful engineering, and dedicate ourselves to helping you build, test, and release your products faster and better. Our core expertise covers four functional areas: Product Lifecycle Management (MCAD, PLM, PDM); Software Lifecycle Management (ALM, DevOps); Cloud for Engineering (Infrastructure, Security); Data Engineering and Insights. Read more about SPK in our Capabilities Statement.

AICPA SOC2 Type 1 Certified

Certified Woman-Owned Business

Atlassian Cloud Migration Specialized

GitLab Professional Services Partner

Top MSP 2025

Top MSP 2024

Top MSP 2023

Top MSP 2022

Top MSP 2021

Top MSP 2020

Top MSP 2019
Our Story
The SPK story is strongly influenced by HP. Both Christine McHale and Steve Kling worked at HP early in their careers in the 1990’s where they learned the “HP Way.” They deeply valued the ethical, employee- and customer-centric way that the company approached business.
HP began to change and each left to work for a small CAD software reseller in 1995. Here, they began to learn about the fascinating world of engineering and product development. Engineering is a discipline that quickly embraced information technology. Engineers began abandoning their drafting tables and calculators for 3D CAD applications running on Unix systems. Christine and Steve were part of that beginning.
After a few years, in 1999, the CAD reseller reorganized, and Steve and Chris decided to pursue independent consulting for a time – Steve in the area of management consulting, and Chris in the area of technical network and systems consulting.
Three things became clear over those formative years. First, the tech world needed companies with a more supportive company culture that put its people, customers, and ethics first, like the HP of old. Focus first on creating the right value with the right culture, and profits would follow. Second, IT was a male dominated world which could benefit from more women, and more women leaders. And third, engineering and product development groups needed specialized IT services from people that truly understood their technology and business, and that were deeply integrated into their work teams.
The opportunities for consulting expanded, until they were faced with a decision – either hire people and build a business, or cut back on what they could handle.
One day in 2003, a woman business leader at one of our key clients pulled Chris aside and asked her again, “Why don’t you hire people and build a business out of this?” Why didn’t they? They decided to take on the challenge.
However… they didn’t want to create “just another IT company.” Chris and Steve wanted to add value in a certain way.
- The company had to strongly value and support its staff.
- The company had to embrace and value diversity.
- The company had to have uncompromising ethics and give back strongly to the community.
- The company would have to work in true partnership with its clients, where the business of the team was deeply understood first, and the technology put to work to advance those business needs.
As a newly-formed company, SPK took on its first large project. They made mistakes, but continued to build. And Chris and Steve built a company that is what they set out to be:
- A company that strongly values its people and shares the profits of the company.
- A company that is uncompromising in its ethics and contributes diligently to chosen charitable causes.
- A company that embraces and values diversity in its people and its clients.
- An IT services company that deeply understands the business of the teams they serve, that closely partners with client teams, and only builds creative technology solutions that achieve their business goals.

Chris McHale
Chief Executive Officer, Co-Founder
She is responsible for the overall operational and financial success of SPK. Chris has over 25 years experience in the technology sector, starting in the trenches as a Unix consultant at Hewlett-Packard. She was the Business Manager of HP’s western professional services organization, before co-founding SPK in 1997. This early experience gave her a deep appreciation of the technology challenges faced by customers and engineers alike. She holds a BA with Honors from University of Virginia, and an MBA from UCLA’s Anderson School of Management.

Steve Kling
Chief Operating Officer, Co-Founder
Steve has spent over 35 years managing and leading professional services organizations at the director and vice president level. Steve’s relentless drive to provide excellent client service comes from his background at Hewlett Packard where he ran the professional services organization in the western United States. Serving as an executive consultant to companies such as Embarcadero Technologies, Visionary Design Systems, AllAdvantage and Hewlett Packard, Steve has helped build and shape world-class technical support organizations.

Carlos Almeida
District Manager & Vice President, Engineering
With over 25 years experience of management in engineering operations, Carlos focuses on bringing together a combination of best practices of software engineering and operational continuous improvement for our customers. He is responsible for setting the technical and business strategy for customer SDLC process engagements and SPK’s SaaS offering. Carlos also drives development of new business opportunities via strategic business relationships with top software ISVs. His prior role included Group Director of Engineering, Cadence Design Systems, leading global organizations focusing on Quality, Release Management, Program Management, Process & Tools Development, IT, and Manufacturing. Carlos also managed key partner technical relationships with customers such as Intel and HP.

Edwin Chung
Vice President, Service Delivery
In his current role, Edwin is responsible for managing a team of Application Engineers that provide architecture, implementation, process reengineering, and ongoing support for MCAD/EE applications. Edwin’s knowledge of FDA Regulations and GxP Quality Systems, combined with his experience using Python for Machine Learning and Data Engineering, enables him to innovate legacy system automations, integrate cloud or IoT products, and increase engineering efficiency. He applies his multiple M.S and B.S. degrees in Biomedical, Electrical and Computer Engineering to architect solutions that solve interdisciplinary business problems. With 20 years of experience, Edwin strives for excellence by prioritizing customer satisfaction and quality.

Mike Solinap
District Manager & Director, Cloud and Infrastructure
With a B.S. in Information Systems Management from University of California, Santa Cruz, Mike has 2 decades of experience in IT, cloud, and data technologies. Mike is highly skilled in architecture, and infrastructure design, including architecting solutions for companies like Johnson & Johnson, Stryker, Ximedica, and many others. Leading client support efforts, Mike has a large scope of responsibility from pre-sales to discovery sessions, creating functional and technical specifications and doing project delivery. Mike has several industry certifications, including those from AWS, and Microsoft.

Sarah Craft
Director of Finance and Contracts
Sarah Craft is currently SPK’s Director of Finance and Contracts. She has worked in finance for over 15 years. Sarah began her career an accountant in a CPA office after receiving her Bachelor of Science from UC Santa Cruz. While there, she held various positions from Registered Tax Preparer to Property Manager. Sarah joined SPK in 2013 as an accountant and helped grow SPK’s finance and contracts function as the company grew. She currently manages all SPK’s Accounting, Finance, and Contracts functions and has developed a deep expertise in Data Analysis and Business Intelligence which she deploys for SPK as well as for some of SPK’s clients. She holds a Post Graduate degree in Data Science and Business Analytics from UT McCombs School of Business and will receive her MBA in 2024.

Michael Roberts
Vice President, Sales and Marketing
Michael comes to SPK with over 20 years of experience in bringing software to market. With a B.S in Business Administration from Methodist University, his industry certifications include those from ICAgile, Cisco, Atlassian, GitLab, CompTIA, Microsoft and the Scaled Agile Framework. With his experience as a technology executive, he’s guided many companies to successful IT and software projects, and become a skilled strategist who transforms strategic plans into workable solutions and benchmarks performance against key operational targets/goals.
Talk with our team
We value speaking with our clients and partners. Interested in joining us? We welcome a conversation.
1999
Steve Kling and Chris McHale begin independent consulting.

2003
SPK and Associates LLC is formed.
First Fortune 100 MedTech client signed.
2007
First three employees are hired.

2011
Cloud Services practice launched.
Became PTC partner.
Became Electric loud partner.
Became AWS partner.

2012
Product Data Management / Product Lifecycle Management Practice launched.
DevOps Practice launched.
Became Microsoft Azure partner.

2017
Attain WBENC (Women-Owned Business from Women's Business Enterprise National Council) and WOSB (Woman-Owned Small Business) certifications.

2018
LicenseMiner launched.
Reached 25 fuly time employees.
10th Fortune 500 MedTech client signed.
Became MasterControl partner.

2019
Scotts Valley HQ established.
Became CloudBees Partner.

2020
SPK vCAD launched.
SolidWorks PDM in the Cloud services launched.
Received Channel Futures MSP 501 award.

2021
Became Atlassian Solution Partner.
Received ChannelFutures MSP 501 award.

2022
Received CloudBees Service Partner of the Year.
Received ChannelFutures MSP 501 award.
Became Greenlight Guru partner.
Became Hello Clerk partner.
Became Revyz partner.

2023
Received ChannelFutures MSP 501 award.
Became yasoon partner.
Became Hawk Ridge Systems partner.
Became GitLab partner.
Became Tempo Software partner.

2024
MSP 501 award
Achieved SOC2 compliance
Became Exalate partner
Became AppFox partner
Became Aptis partner
Became OpsHub partner
Became Botable partner

2025
MSP 501 award
Achieved Atlassian Cloud Specialization
Achieves GitLab Professional Services Partner
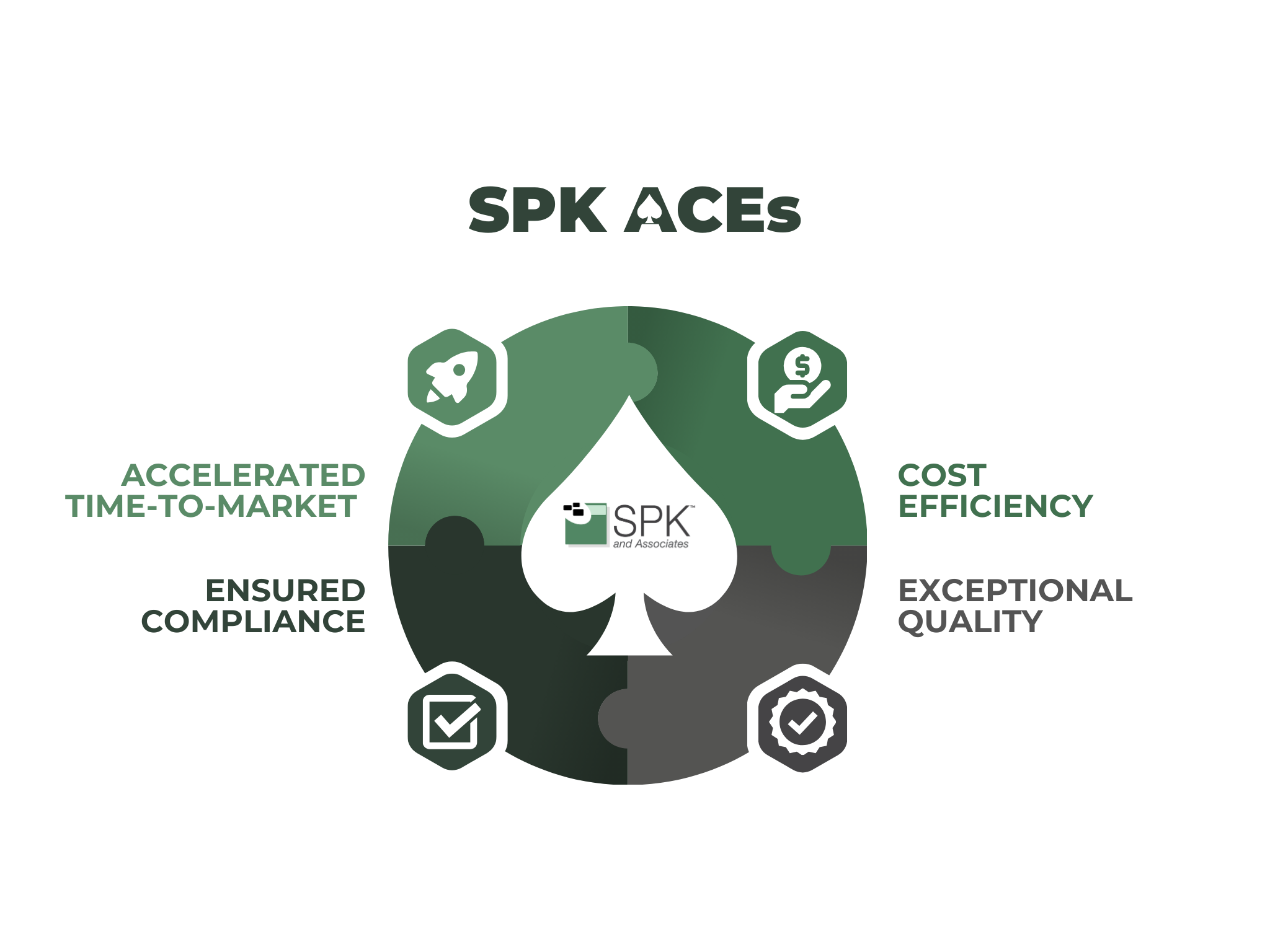
What is SPK ACEs?
SPK is proud to have aided our customers for over 20 years. Our main goal has always been accelerating products to market while ensuring cost-efficiency, compliance, and quality. If you would like to save time and money while increasing efficiency and compliance, SPK can help. Learn more here.
What our customers say about us
Our company had a plan for cloud implementation but we weren’t sure if we were making the right decisions on a few areas, including security. When we engaged SPK, not only did they validate our plan, they provided so much more insight than we originally thought about and helped revamp our cloud plan for the better.
We needed Atlassian implementation help and SPK was great . The fit our budget, and helped us implement a system that worked for our team. I would recommend SPK for others needing to implement a Jira Service Desk system. In particular, Steve L. was great.
I loved working with SPK. I got what I needed, they provided the right information at the perfect time. I was even impressed that I didn’t have to ask them to do things like work within our policies. They asked me before I had the chance. Their consultants are professional and really know how to work with SolidWorks.
Careers @ SPK and Associates
SPK is always looking for highly motivated individuals who are searching for the very best place to work and express their technical talents. We commonly hire field support engineers, software developers, project managers, mechanical CAD experts and other technical disciplines.