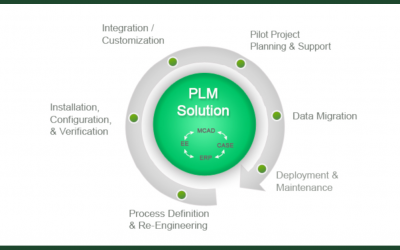
Developing new and innovative products is essential for companies to survive and thrive -- however safety can never take a backseat to innovation. That is why many companies, like those in the Medical Devices, Aeronautics and Automotive industries, have strict...
Medical Device Engineering
How PLM Enables Innovation Without Risking Compliance
As technology has advanced, it's become more complex -- and with complexity comes the issue of tracking and monitoring the different processes and their corresponding data. In non-critical applications, the extent of this reporting is often minimal. However, in...
Blog: Leveraging PTC’s Integrity Platform for IEC 62304 Compliance
SPK and Associates leverage PTC’s Integrity platform to help Medical Device companies develop software efficiently while achieving IEC 62304 compliance.
A Review of FDA 483 Observations – Top Med Device Issues Sited & Proper Response
This article reviews what an FDA 483 Observation looks like, some of the more common issues flagged in medical device companies, and how to respond.
Design Output: A Review of 21 C.F.R. §820.30(d) and FDA Warning Letter Trends
Design Output: A Review of 21 C.F.R. §820.30(d) and FDA Warning Letter Trends
CAPA: A Review of 21 C.F.R. §820.100 and FDA Warning Letter Trends
SPK and Associates routinely review warning letters to help our clients stay in step with FDA trends. One of the problem areas most often cited in company audits continue to be the CAPA system/program. This month we will take a look at some of the latest FDA...