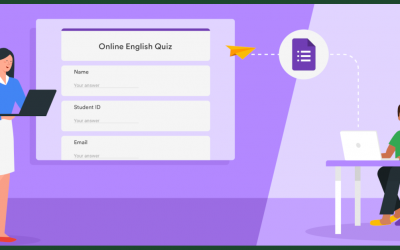
Have you ever sent out an email with a bunch of important questions and received a reply that began with "my answers are in blue". Have you then found yourself combing through your large email searching for those answers only to discover that your email provider...
Data Engineering
How To Upgrade the Feature Level in IBM Rational ClearQuest
Sometimes finding the information you want for IBM Rational ClearQuest can be a challenge -- and when you find something, it can be that the level of detail needed is not present. That's why we at SPK and Associates started this series of technical how to guides to...
Troubleshooting PTC Creo Elements Pro/Engineer Performance Using Procmon.exe
Everyone knows that troubleshooting “slow performance” is one of the most challenging tasks we have as IT services providers, but is also one of the best ways to improve productivity (and morale!). Although replacing hardware and buying more expensive computers can...
Medical Device Interoperability: A $30B opportunity?
Greater medical device interoperability and the adoption of commonly accepted standards could save the US in excess of $30B, suggests a West Health Institute report published in March. Lack of device interoperability creates significant waste and risk to patient...
Tackling Email Archiving Regulations
Whether or not your in a heavily regulated industry, having a solid email archiving solution is an absolute necessity. Email’s importance in the workplace for the past decade-and-a-half is similar to the need of having a dial tone in years past. In addition, email is...
Keeping your Ego in check to Maximize your Toolbox.
I don't usually read autobiographies, but recently a close friend of mine suggested that I read "Surely you're joking, Mr. Feynman!" Being bored with the book I was currently reading, I decided to give it a go. In it there is a chapter title "A Different Box of...
PLM in the Cloud: Computer System Validation in FDA Regulated Industries
Product lifecycle management (PLM) systems have evolved from being custom-built, on-premise applications to cloud-based, off-the-shelf solutions. As adoption for PLM in the cloud increases, system validation approaches in FDA/GXP regulated industries have had to...
ROHS 2 for Medical Devices: Are You Ready?
As of July 22, 2014, the RoHS (Restriction of Hazardous Substances) Directive must be observed for first time distribution of all medical devices to the full extent. Furthermore, all products with a CE marking must also be RoHS-compliant. ROHS 2 Compliance Changes at...
Automatically Scraping Webpages using Python 2.7
In today’s Internet, it takes specific skills to efficiently find the “Data You Want” inside of the “Data You’re Given”. I was reminded of this the other day watching a colleague struggle through data collection, clicking buttons and getting diverted by advertisements...
The FDA UDI Rule: 5 Things You Need to Know
The release of the FDA final rule on Unique Device Identification (UDI) is expected this summer. Here are five things you need to know: 1. What is the UDI Rule? In July 2012, the FDA proposed a rule requiring medical device manufacturers to label their products with...
Integrating Medical Device Product Development with the Quality Management System
A critical business challenge for medical device manufacturers as they scale is getting products to market quickly while supporting existing products and meeting FDA Quality System Regulation (21-CFR-820) requirements. To achieve this effectively, Product Development...
5 Reasons Why You Should Convert a Physical Machine to a Virtual Machine
It's common knowledge that virtual infrastructure and virtual computing is becoming the standard rather than the exception. The pros of virtual computing far outweigh the cons, and as a result, more and more physical machines are being turned into virtual machines. In...